A RESPECTED STATIN FORMULATION WITH FLEXIBLE ADMINISTRATION OPTIONS DESIGNED FOR LTC RESIDENTS WHO CANNOT OR WILL NOT SWALLOW SOLID MEDICATION FORMS
Ezallor Sprinkle™ eliminates the uncertainty associated with crushing and administering crushed tablets.1,2 Its novel formulation is in line with current LTC guidelines that specify “minimal manipulation” of medications.3
Ezallor Sprinkle™ Bioavailability Proven Equivalent
to Crestor® (rosuvastatin calcium)4

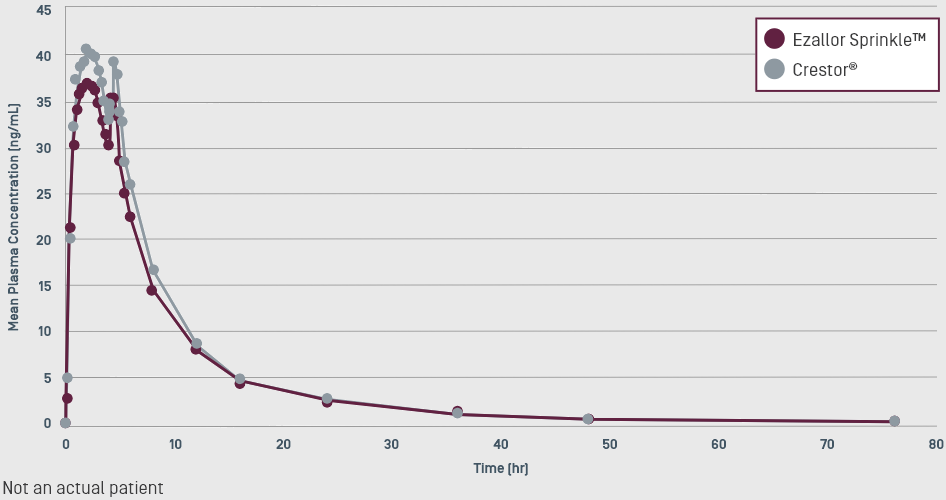
Ezallor Sprinkle™ was approved through the 505(b)(2) regulatory pathway4
The 505(b)(2) regulatory pathway is an abbreviated process that allows manufacturers to submit some product data, such as safety and efficacy data, that came from another manufacturer or researcher.5
Candidates for 505(b)(2) approval include drugs with changes in dosage form, strength, formulation, dosing regimen, or route of administration5
The 505(b)(2) submission demonstrated bioequivalence between Ezallor Sprinkle™ and the listed drug, Crestor® tablets. This was achieved through 2 pivotal bioequivalence (BE) studies—one under fasting conditions and one under fed conditions—to assess the BE between rosuvastatin 40-mg capsules (both intact and sprinkling the contents over applesauce) versus Crestor® 40-mg tablets.
Ezallor Sprinkle™ was subsequently approved by the FDA for the following indications:
INDICATIONS AND USAGE
Ezallor Sprinkle™ (rosuvastatin) capsules are indicated as an:
Adjunctive therapy to diet for the treatment of adult patients with hypertriglyceridemia
Adjunct to diet for the treatment of adult patients with primary dysbetalipoproteinemia (Type III hyperlipoproteinemia)
Adjunctive therapy to other lipid-lowering treatments (e.g., LDL apheresis) or alone if such treatments are unavailable to reduce LDL-C, total-C, and ApoB in adult patients with homozygous familial hypercholesterolemia
LIMITATIONS OF USE
Ezallor Sprinkle™ has not been studied in Fredrickson Type I and V dyslipidemias. Ezallor Sprinkle™ is indicated only for use in patients 18 and older.

Based on active ingredients present in existing approved drug products... 505(b)(2) requires manufacturers create a “bridge” between what is already known about the previously approved reference drug and the novel aspects of the new product.5
A Caution About Geriatric Use:
Ezallor Sprinkle™ should be prescribed with caution in patients with predisposing factors for myopathy (e.g., age ≥65 years, inadequately treated hypothyroidism, renal impairment). The risk of myopathy during treatment with Ezallor Sprinkle™ may be increased with concurrent administration of some other lipid-lowering therapies (fibrates or niacin), gemfibrozil, cyclosporine, lopinavir/ritonavir, atazanavir/ritonavir, or simeprevir. Please see Important Safety Information and Full Prescribing Information.

Discover how Ezallor Sprinkle™ utilizes a unique sprinkle technology
WARNINGS AND PRECAUTIONS
Liver Enzyme Abnormalities: It is recommended that liver enzyme tests be performed before the initiation of Ezallor Sprinkle™, and if signs or symptoms of liver injury occur. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including rosuvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with Ezallor Sprinkle™, promptly interrupt therapy. If an alternate etiology is not found, do not restart Ezallor Sprinkle™. Ezallor Sprinkle™ should be used with caution in patients who consume substantial quantities of alcohol and/or have a history of chronic liver disease. Active liver disease, which may include unexplained persistent transaminase elevations, is a contraindication to the use of Ezallor Sprinkle™.
IMPORTANT SAFETY INFORMATION
INDICATIONS AND USAGE
Ezallor Sprinkle™ (rosuvastatin) capsules are indicated as an:
Adjunctive therapy to diet for the treatment of adult patients with hypertriglyceridemia
Adjunct to diet for the treatment of adult patients with primary dysbetalipoproteinemia (Type III hyperlipoproteinemia)
Adjunctive therapy to other lipid-lowering treatments (e.g., LDL apheresis) or alone if such treatments are unavailable to reduce LDL-C, total-C, and ApoB in adult patients with homozygous familial hypercholesterolemia
LIMITATIONS OF USE
Ezallor Sprinkle™ has not been studied in Fredrickson Type I and V dyslipidemias. Ezallor Sprinkle™ is indicated only for use in patients 18 and older.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Patients with a known hypersensitivity to any component of this product. Hypersensitivity reactions including rash, pruritus, urticaria, and angioedema have been reported with rosuvastatin.
Patients with active liver disease, which may include unexplained persistent elevations of hepatic transaminase levels.
Pregnancy: advise females of reproductive potential to use effective contraception during treatment with Ezallor Sprinkle™.
Lactation: limited data indicate that rosuvastatin is present in human milk. Because statins have the potential for serious adverse reactions in nursing infants, women who require Ezallor Sprinkle™ treatment should not breastfeed their infants.
WARNINGS AND PRECAUTIONS
Skeletal Muscle Effects: Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. These risks can occur at any dose level, but are increased at the highest dose (40 mg). Ezallor Sprinkle™ should be prescribed with caution in patients with predisposing factors for myopathy (e.g. aged ≥65 years, inadequately treated hypothyroidism, renal impairment). The risk of myopathy during treatment with Ezallor Sprinkle™ may be increased with concurrent administration of gemfibrozil, some other lipid-lowering therapies (other fibrates or niacin), cyclosporine, darolutamide, regorafenib, atazanavir/ritonavir, lopinavir/ritonavir, simeprevir or combination of sofosbuvir/velpatasvir/voxilaprevir, dasabuvir/ombitasvir/paritaprevir/ritonavir, elbasvir/grazoprevir, sofosbuvir/velpatasvir, glecaprevir/pibrentasvir, all combinations with ledipasvir (including ledipasvir/sofosbuvir). Ezallor Sprinkle™ therapy should be discontinued if markedly elevated CK levels occur or myopathy is diagnosed or suspected.
Immune-Mediated Necrotizing Myopathy (IMNM): There have been rare reports of IMNM, an autoimmune myopathy, associated with statin use. IMNM is characterized by proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; positive anti-HMG-CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents.
Liver Enzyme Abnormalities: It is recommended that liver enzyme tests be performed before the initiation of Ezallor Sprinkle™, and if signs or symptoms of liver injury occur. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including rosuvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with Ezallor Sprinkle™, promptly interrupt therapy. If an alternate etiology is not found, do not restart Ezallor Sprinkle™. Ezallor Sprinkle™ should be used with caution in patients who consume substantial quantities of alcohol and/or have a history of chronic liver disease. Active liver disease, which may include unexplained persistent transaminase elevations, is a contraindication to the use of Ezallor Sprinkle™.
Concomitant Coumarin Anticoagulants: Caution should be exercised when anticoagulants are given in conjunction with Ezallor Sprinkle™ because of its potentiation of the effect of coumarin-type anticoagulants in prolonging the prothrombin time/INR. In patients taking coumarin anticoagulants and Ezallor Sprinkle™ concomitantly, INR should be determined before starting Ezallor Sprinkle™ and frequently enough during early therapy to ensure that no significant alteration of INR occurs.
Proteinuria and Hematuria: Dipstick-positive proteinuria and microscopic hematuria were observed among patients treated with rosuvastatin. These findings were more frequent in patients taking rosuvastatin 40 mg, though it was generally transient and was not associated with worsening renal function. Although the clinical significance of this finding is unknown, dose reduction should be considered for patients on Ezallor Sprinkle™ therapy with unexplained persistent proteinuria and/or hematuria during routine urinalysis testing.
Endocrine Effects: Increases in HbA1c and fasting serum glucose levels have been reported with statins, including rosuvastatin. Based on clinical trial data with rosuvastatin, in some instances these increases may exceed the threshold for the diagnosis of diabetes mellitus.
ADVERSE REACTIONS
In the controlled clinical trials database, the most common adverse reactions were headache, myalgia, abdominal pain, asthenia, and nausea.
There have been rare reports of immune-mediated myopathy associated with statin use. There have been rare postmarketing reports of cognitive impairment (e.g. memory loss, forgetfulness, amnesia, memory impairment, and confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally non-serious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Full Prescribing Information.
References: 1. Ezallor Sprinkle™ [prescribing information]. Cranbury, NJ: Sun Pharmaceutical Industries, Inc., 2020. 2. Thong MW, Manrique YJ, Steadman KJ. Drug loss while crushing tablets: comparison of 24 tablet crushing devices. PLoS ONE. 2018;13(3):e0193683. 3. Joint Commission International. Joint Commission International Accreditation Standards for Long-Term Care. 1st ed. Oak Brook, IL: Joint Commission International; 2012. 4. Data on file. Sun Pharmaceutical Industries Inc., Princeton, NJ. 5. Camargo Pharmaceutical Services. What is 505(b)(2)? A guide to the 505(b)(2) regulatory pathway. https://camargopharma.com/what-is-505b2. Accessed May 8, 2019.
All trademarks are property of their respective owners.
IMPORTANT SAFETY INFORMATION
INDICATIONS AND USAGE
Ezallor Sprinkle™ (rosuvastatin) capsules are indicated as an:
Adjunctive therapy to diet for the treatment of adult patients with hypertriglyceridemia
Adjunct to diet for the treatment of adult patients with primary dysbetalipoproteinemia (Type III hyperlipoproteinemia)
Adjunctive therapy to other lipid-lowering treatments (e.g., LDL apheresis) or alone if such treatments are unavailable to reduce LDL-C, total-C, and ApoB in adult patients with homozygous familial hypercholesterolemia
LIMITATIONS OF USE
Ezallor Sprinkle™ has not been studied in Fredrickson Type I and V dyslipidemias. Ezallor Sprinkle™ is indicated only for use in patients 18 and older.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Patients with a known hypersensitivity to any component of this product. Hypersensitivity reactions including rash, pruritus, urticaria, and angioedema have been reported with rosuvastatin.
Patients with active liver disease, which may include unexplained persistent elevations of hepatic transaminase levels.
Pregnancy: advise females of reproductive potential to use effective contraception during treatment with Ezallor Sprinkle™.
Lactation: limited data indicate that rosuvastatin is present in human milk. Because statins have the potential for serious adverse reactions in nursing infants, women who require Ezallor Sprinkle™ treatment should not breastfeed their infants.
WARNINGS AND PRECAUTIONS
Skeletal Muscle Effects: Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. These risks can occur at any dose level, but are increased at the highest dose (40 mg). Ezallor Sprinkle™ should be prescribed with caution in patients with predisposing factors for myopathy (e.g. aged ≥65 years, inadequately treated hypothyroidism, renal impairment). The risk of myopathy during treatment with Ezallor Sprinkle™ may be increased with concurrent administration of gemfibrozil, some other lipid-lowering therapies (other fibrates or niacin), cyclosporine, darolutamide, regorafenib, atazanavir/ritonavir, lopinavir/ritonavir, simeprevir or combination of sofosbuvir/velpatasvir/voxilaprevir, dasabuvir/ombitasvir/paritaprevir/ritonavir, elbasvir/grazoprevir, sofosbuvir/velpatasvir, glecaprevir/pibrentasvir, all combinations with ledipasvir (including ledipasvir/sofosbuvir). Ezallor Sprinkle™ therapy should be discontinued if markedly elevated CK levels occur or myopathy is diagnosed or suspected.
Immune-Mediated Necrotizing Myopathy (IMNM): There have been rare reports of IMNM, an autoimmune myopathy, associated with statin use. IMNM is characterized by proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; positive anti-HMG-CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents.
Liver Enzyme Abnormalities: It is recommended that liver enzyme tests be performed before the initiation of Ezallor Sprinkle™, and if signs or symptoms of liver injury occur. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including rosuvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with Ezallor Sprinkle™, promptly interrupt therapy. If an alternate etiology is not found, do not restart Ezallor Sprinkle™. Ezallor Sprinkle™ should be used with caution in patients who consume substantial quantities of alcohol and/or have a history of chronic liver disease. Active liver disease, which may include unexplained persistent transaminase elevations, is a contraindication to the use of Ezallor Sprinkle™.
Concomitant Coumarin Anticoagulants: Caution should be exercised when anticoagulants are given in conjunction with Ezallor Sprinkle™ because of its potentiation of the effect of coumarin-type anticoagulants in prolonging the prothrombin time/INR. In patients taking coumarin anticoagulants and Ezallor Sprinkle™ concomitantly, INR should be determined before starting Ezallor Sprinkle™ and frequently enough during early therapy to ensure that no significant alteration of INR occurs.
Proteinuria and Hematuria: Dipstick-positive proteinuria and microscopic hematuria were observed among patients treated with rosuvastatin. These findings were more frequent in patients taking rosuvastatin 40 mg, though it was generally transient and was not associated with worsening renal function. Although the clinical significance of this finding is unknown, dose reduction should be considered for patients on Ezallor Sprinkle™ therapy with unexplained persistent proteinuria and/or hematuria during routine urinalysis testing.
Endocrine Effects: Increases in HbA1c and fasting serum glucose levels have been reported with statins, including rosuvastatin. Based on clinical trial data with rosuvastatin, in some instances these increases may exceed the threshold for the diagnosis of diabetes mellitus.
ADVERSE REACTIONS
In the controlled clinical trials database, the most common adverse reactions were headache, myalgia, abdominal pain, asthenia, and nausea.
There have been rare reports of immune-mediated myopathy associated with statin use. There have been rare postmarketing reports of cognitive impairment (e.g. memory loss, forgetfulness, amnesia, memory impairment, and confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally non-serious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see Full Prescribing Information.



